The journey of a metal part doesn't end when it comes off the stamping press. The final metal surface finishing is a critical step that defines its appearance, durability, and performance. Among the most common choices, the anodizing vs. electroplating debate often arises. Both processes create protective and decorative layers, but they work in fundamentally different ways. This practical guide will break down the science, benefits, and best applications for each, helping you choose the perfect finish for your product.

What is Anodizing? The Science of Aluminum Protection
What is anodizing? Anodizing is not a coating that is applied to the surface; it's an electrochemical process that converts the metal surface itself into a decorative, durable, and corrosion-resistant anodic oxide finish. It is integral to the part, not just a layer on top.
The Anodizing Process Explained: An Electrochemical Conversion
The process of anodizing aluminum involves submerging the aluminum part into an acid electrolyte bath and passing an electric current through it. The aluminum part acts as the anode (positive electrode), causing oxygen to be released at the surface. This oxygen combines with the aluminum to form a hard, highly controlled oxide layer, which is an electrochemical conversion of the base metal.
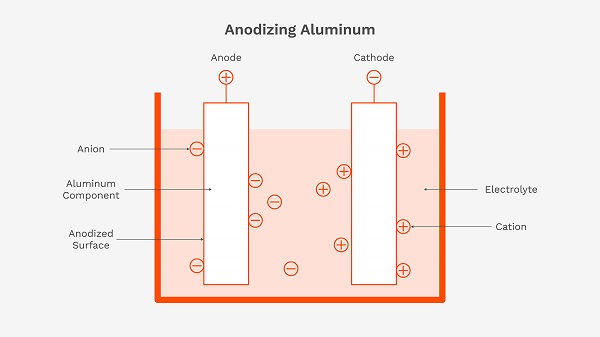
Key Advantages: Durability, Color Options, and Corrosion Resistance
The primary benefit of anodizing is its exceptional hardness and wear resistance. The anodic layer is much harder than the raw aluminum. It also provides excellent corrosion resistance. Furthermore, the porous nature of the oxide layer allows it to be dyed in a wide variety of colors, making it a popular choice for a vibrant decorative finish.
Best Applications for Anodizing
Anodizing is almost exclusively used for aluminum. It is the ideal choice for applications like:

- High-end electronic enclosures (e.g., smartphones, laptops)
- Architectural components and window frames
- Automotive trim and performance parts
- Cookware and sporting goods
What is Electroplating? Adding a New Metal Layer
What is electroplating? Unlike anodizing, the electroplating process involves depositing a thin layer of a different metal onto the surface of a part (the substrate). It is an additive process that applies a new material to the original component.
The Electroplating Process Explained: Using Electric Current
In electroplating, the part to be coated is placed in a chemical bath containing dissolved ions of the plating metal (like nickel, chromium, or zinc). When a direct electric current is applied, the metal ions are drawn to the part and deposited onto its surface, forming a thin, uniform metallic coating. This process is often used for plating on steel.
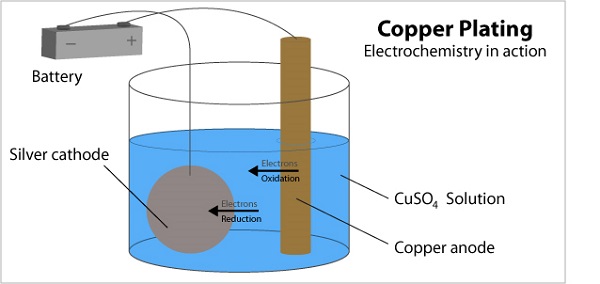
Key Advantages: Conductivity, Decorative Appeal, and Versatility
Electroplating's main advantage is its versatility. It can be applied to a wide range of metals, not just aluminum. It can be used to achieve specific functional properties, such as enhanced electrical conductivity (gold or silver plating) or a brilliant, mirror-like decorative finish (chrome plating).
Best Applications for Electroplating
Electroplating is used across countless industries for both functional and aesthetic purposes. Common applications include:
- Corrosion protection for steel automotive parts (zinc plating)
- Decorative and durable bathroom and kitchen fixtures (chrome plating)
- Electrical connectors and contacts (gold or silver plating)
- Jewelry and fashion accessories
Head-to-Head Comparison: Anodizing vs. Electroplating
To make the best choice, let's compare these two processes directly across several key factors.
Material Compatibility: Aluminum's Best Friend vs. Metal's Versatile Coat
This is the most critical difference. Anodizing is a process specifically for aluminum and a few other non-ferrous metals like titanium. Electroplating, on the other hand, is extremely versatile and can be applied to a vast range of metals, including steel, iron, copper, and brass.
Durability and Wear Resistance
Which finish is more durable? Anodizing creates a very hard, ceramic-like surface that is integral to the part, offering exceptional scratch and wear resistance. The durability of an electroplated layer depends entirely on the metal being deposited. Hard chrome plating is extremely durable, while a decorative gold plate may be softer.
Appearance and Decorative Options
Both offer excellent decorative options. Anodizing produces a unique, deep metallic sheen and can be dyed in many colors. Electroplating is capable of producing a much wider range of appearances, from a brilliant, reflective mirror finish (chrome) to the rich luster of gold or the industrial look of zinc.
Cost Analysis: A Look at the Overall Price
A direct cost analysis can be complex. Generally, standard anodizing can be more cost-effective than some multi-layer electroplating processes. However, the cost is heavily influenced by the size of the part, the complexity of the process, and the specific metals or colors involved. It is always best to get a quote for your specific needs.
Making the Right Choice for Your Custom Stamped Parts
Your choice ultimately comes down to your base material and desired outcome.
When to Choose Anodizing for Your Aluminum Parts
You should choose anodizing aluminum parts when your product is made of aluminum and your primary requirements are high durability, excellent wear resistance, and the option for vibrant, long-lasting color.
When to Choose the Electroplating Process for Steel or Iron Parts
You should choose the electroplating process when your base material is steel, iron, or another metal, or when you require a specific surface property that only another metal can provide, such as a mirror-like shine or high electrical conductivity. For a detailed consultation, you can contact Pengce Metal's experts.
The Right Finish for a Perfect Product
In the anodizing vs. electroplating showdown, there is no single winner—only the right tool for the right job. Understanding the fundamental differences in their processes, material compatibility, and resulting properties is key to creating a product that not only functions perfectly but also looks and lasts as intended. What factors are most important to you when choosing a surface finish? Durability, cost, or appearance? Let us know in the comments!
Your Metal Surface Finishing Questions Answered
Can you anodize steel or electroplate aluminum?You cannot anodize steel, as the process relies on the specific way aluminum forms an oxide layer. You can electroplate aluminum, but it requires a special pre-treatment process (like applying a zincate coating) to ensure the plated layer adheres properly.
Does anodizing add thickness to a part?Yes, it does. During anodizing, some of the base material is converted to oxide, and the layer also grows outwards from the surface. This dimensional change is predictable but must be accounted for in the initial design, especially for parts with tight tolerances.
Which is better for corrosion protection in a salt-spray test?Both can offer excellent protection, but it depends on the specifics. A properly sealed, thick anodized layer performs very well. Similarly, a thick, properly applied zinc or nickel-chrome electroplated layer on steel is designed specifically to withstand harsh salt-spray conditions. The best choice depends on the base material and the specific test requirements.
How do I specify the desired finish when requesting a quote?When requesting a quote, be as specific as possible. For anodizing, specify the type (e.g., Type II), color, and required thickness. For electroplating, specify the plating material (e.g., nickel, zinc, chrome), the required thickness, and any underlying layers (e.g., copper flash). Providing these details will help us give you an accurate quote. Submit your detailed request today.




